Events calendar
Workshop on Stress Corrosion Cracking and Hydrogen Embrittlement of Concrete Reinforcing Steels
RILEM Technical Committee 293-CCH virtual workshop
It is a well-known fact that corrosion is the main cause of structure degradation. A particular case of corrosion of the steel embedded in concrete is the Environment Assisted Cracking (EAC), which can appear in prestressed structures. The Environment Assisted Cracking takes into account two main phenomena: Stress Corrosion Cracking (SCC), and Hydrogen Embrittlement (HE), that can appear together and reduces substantially the structure safety.
Stress Corrosion Cracking is usually defined as cracking owing to a process involving joint corrosion and straining of a metal because of residual or applied stress. The stresses required are tensile and essentially static. So, stress corrosion cracking is driven by the local environment, mechanics, and materials characteristics, and the chemical, electrochemical, and transport processes.
From a scientific point of view, the different mechanisms involved on Stress Corrosion Cracking have not been satisfactorily explained. Numerous mechanisms have been proposed to understand the brittle failure of metals under stress, but only some of them, four specially, are considered to be relevant: i) Mechanism of Anodic Dissolution, ii) Film-induced Cleavage Model, iii) Surface Mobility SCC Mechanism, iv) Environmentally Enhanced Plasticity.
Coherent with the lack of agreement in the type of mechanism that operates, there is no general agreement on testing methods for the study of the Stress Corrosion Cracking.
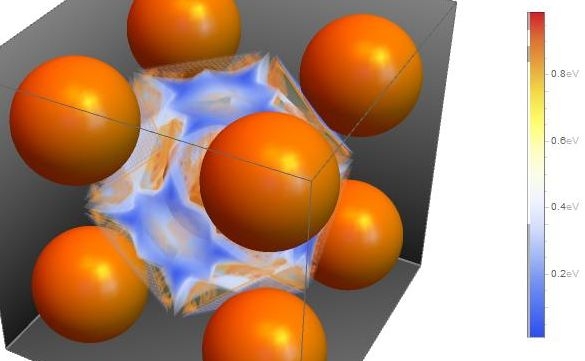
On the other hand, Hydrogen Embrittlement is believed to be one of the main reasons for cracking of steel structures under stress. Hydrogen Embrittlement can occur even during the Stress Corrosion Cracking process. Hydrogen is generated on the steel surface in the cathodic corrosion reaction and is incorporated to the iron lattice. High strength steels often include a ferritic core made of alpha-iron (body-centered-cubic lattice, bcc).
To control and prevent the cracking of steel it is necessary to understand the chemical and physical properties of hydrogen inside bcc iron. There are some experimental studies about the hydrogen transport, diffusivity and trapping which define the crack propagation rate according to electrochemical and mechanical conditions. From the theoretical point of view, there are several studies at different scales: abinitio calculations, molecular dynamics calculations and finite element calculations.
Main topics
Stress Corrosion Cracking of construction steel
- Past experiences
- Mechanisms of SCC
- Protection and assessment of post-tensioned/prestress tendons
Hydrogen Embrittlement of construction steel
- Mechanisms of HE
- Modelling of crack propagation
Normative tests to prestressing steels
Case studies
- SCC of carbon steel radioactive waste
- SCC in ground anchors
- HE in structural steels: fracture and fatigue
- Structural integrity of bridge straps
- HE of galvanized tendons
- SCC of stainless steel tendons
- Failures of prestressed tendons
No comment
Log in to post comment. Log in.